The Progress With Time Of A Reaction System Is Depicted
The progress with time of a reaction system is depicted. There are four main reaction mechanisms based on binding order between enzyme and substrate and production order of products. D The reaction will shift in the direction of reactants. The reaction time is very well defined the uncertainty is 510 ms which is the estimated time for the reaction to be quenched in the cryosolvent and is a function of the length of the aging hose and the flow speed for reaction transfer through the aging hose.
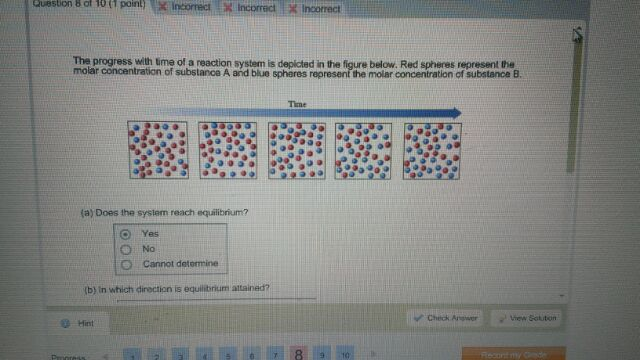
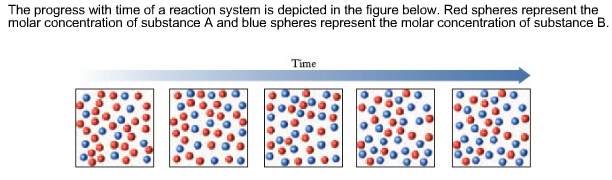
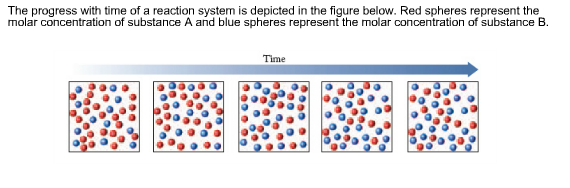
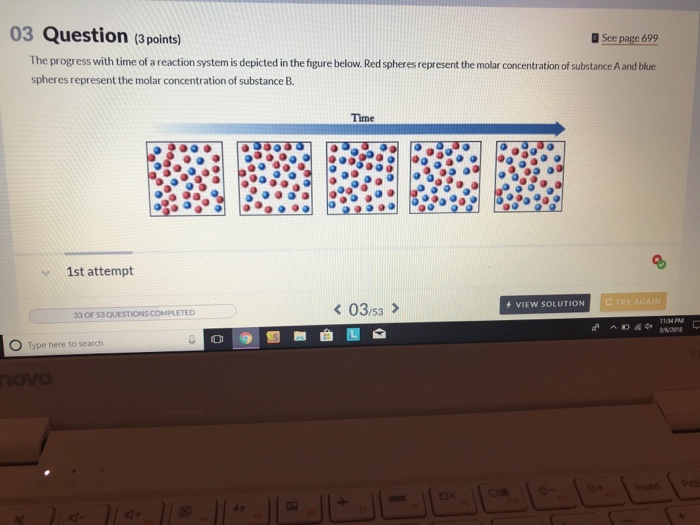
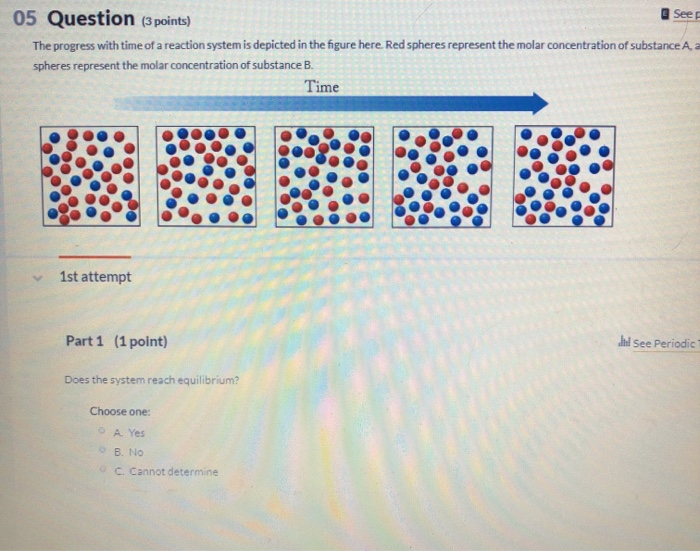
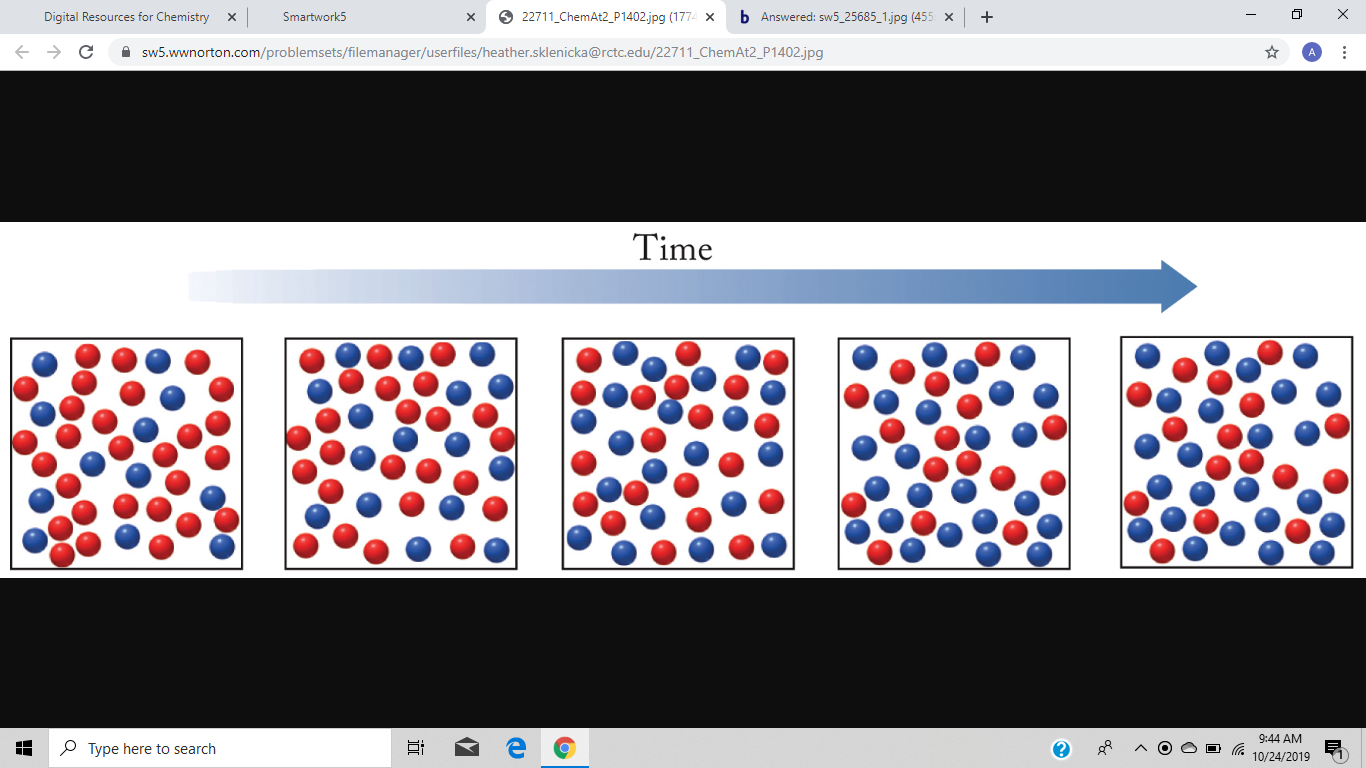
Transcribed image text. If we look at the full time course of the reaction we notice something interest-ing. Red spheres represent the molar concentration of substance A and blue.
The progress with time of a reaction system is depicted in the figure here. For TLC the time off the belt is the time the compounds are adsorbed onto the stationary phase. 2b it is presumed that the APS reaction proceeds via the intermediacy of an alkynyl copper species 18 generated from 8 by deprotonation and then an electrophilic species 19.
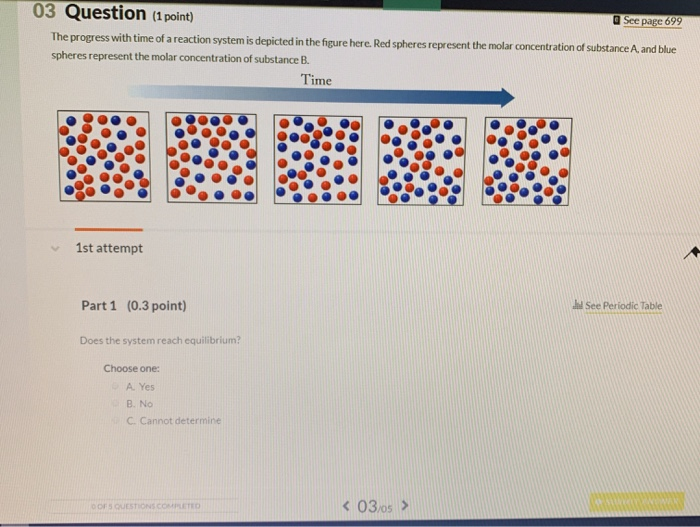
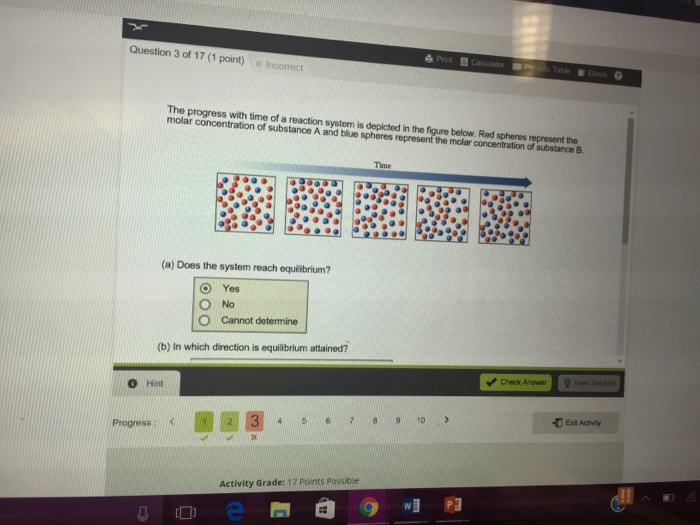
Time 1st attempt Part 1 03 point See Periodic Table Does the system reach equilibrium. To be metabolized glucose must be converted to glucose six phosphate. The system recovers the clamping fixed point with 0 pioneers in the low-noise or high-coupling regimes nontrivial fixed points for large-noise or low-coupling regimes and a uniformly positive flow chain reaction and noise-induced synchronization for intermediate values red spherelike surface.
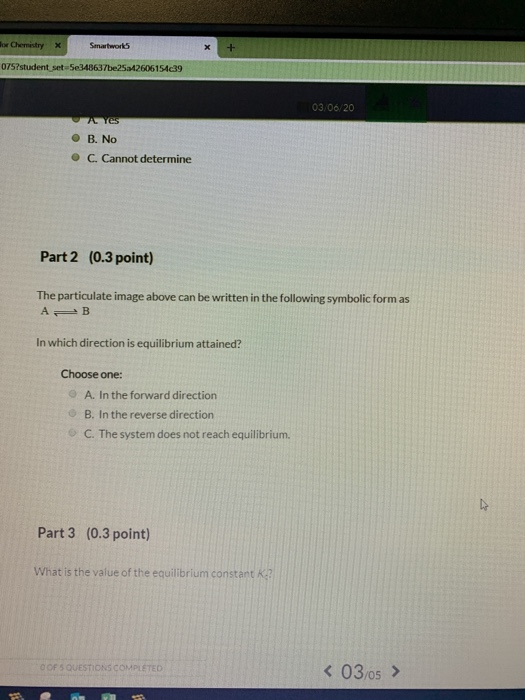
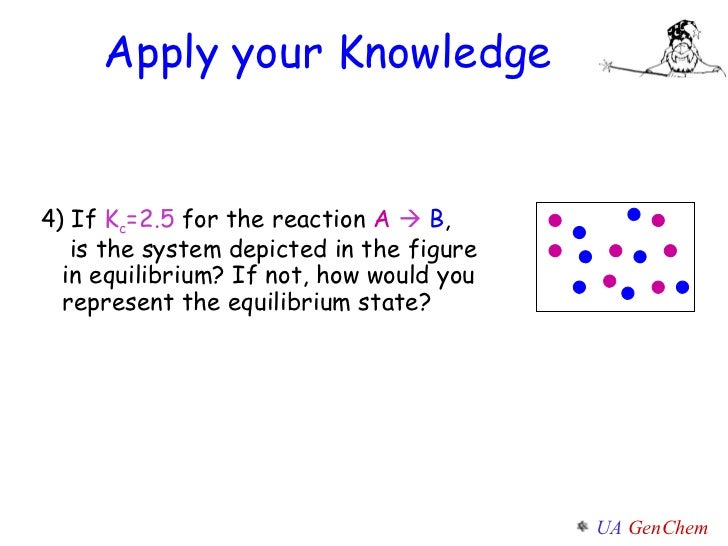
When the energy profile of such a reaction is depicted it is convenient to explicitly state the prevailing conditions state of the system. For this equilibrium got a plus B an equilibrium with a B. 1 ordered Bi Bi mechanism 2 Theorell-chance mechanism 3 rapid equilibrium random Bi Bi mechanism and 4 ping pong Bi Bi mechanism.
Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers. In order to understand how the concentrations of the species in a chemical reaction change with time it is necessary to integrate the rate law which is given as the time-derivative of one of the concentrations to find out how the concentrations change over time. Determine the velocity in ms of the object during the last four seconds OR last six seconds.
There are five molecules of a B 11 molecules of a 12 molecules of B and this would give us a quotient of 00 38 eso. Red spheres represent the molar concentration of substance A and blue spheres represent the molar concentration of.
The motion of an object is depicted by the position-time graph below.
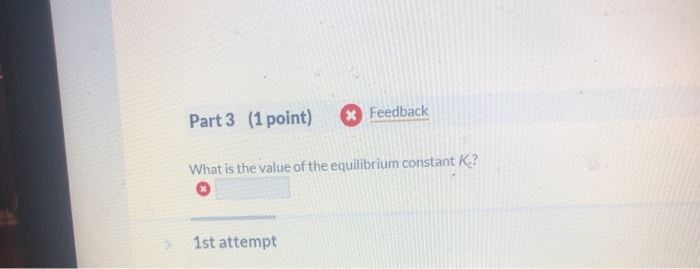
However the phosphorylation of glucose to form glucose six phosphate is endergonic with a positive delta G. Determine the velocity in ms of the object during the last four seconds OR last six seconds. When the energy profile of such a reaction is depicted it is convenient to explicitly state the prevailing conditions state of the system. Methods in Enzymology 2002. For this equilibrium got a plus B an equilibrium with a B. In order to understand how the concentrations of the species in a chemical reaction change with time it is necessary to integrate the rate law which is given as the time-derivative of one of the concentrations to find out how the concentrations change over time. Plan The average rate is given by the change in concentration A divided by the change in time t. C No change will occur since SO3 is not included in the equilibrium expression. Include a numerical answer no units accurate to the second decimal place.
A compound monitor the progress of a reaction determine the solvent composition for preparative separations and analyze the fractions obtained from column chromatography. The progress with time of a reaction system is depicted in Figure mathrmP 142 Red spheres represent the molar concentration of substance A and blue spheres represent the molar concentration of substance B. Red spheres represent the molar concentration of substance A and blue spheres represent the molar concentration of. 234x10 InA 42x10- Timesec Half life of this reaction is. When the energy profile of such a reaction is depicted it is convenient to explicitly state the prevailing conditions state of the system. Plan The average rate is given by the change in concentration A divided by the change in time t. Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.













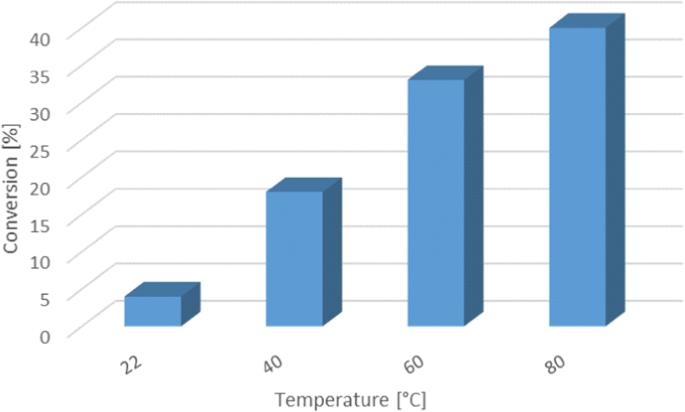


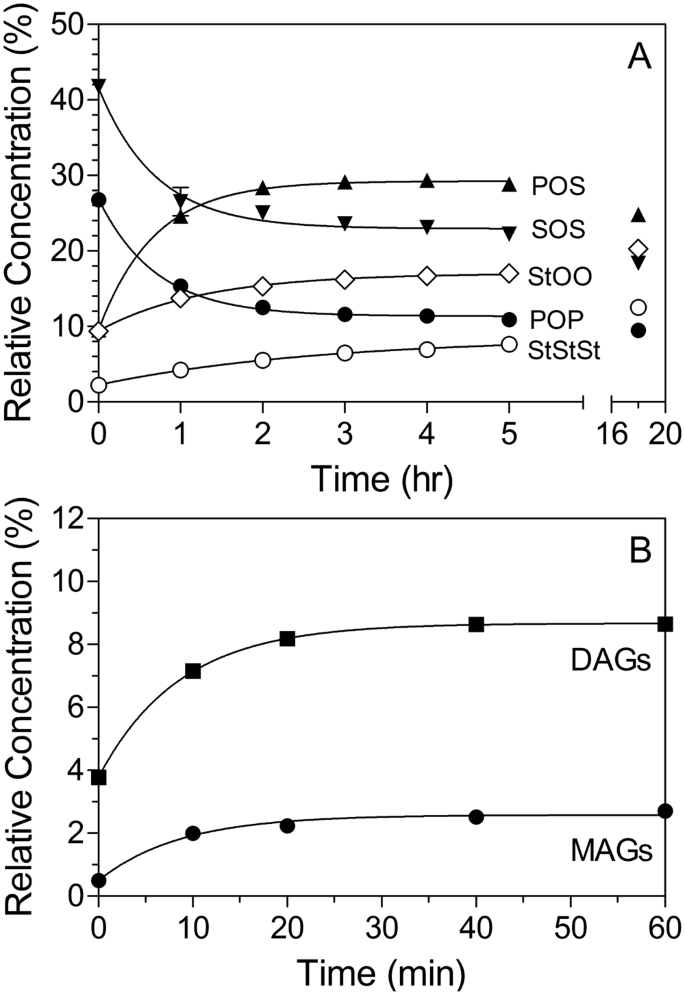


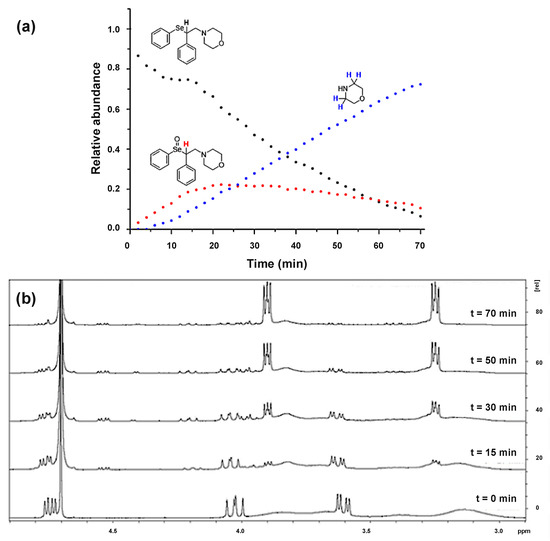

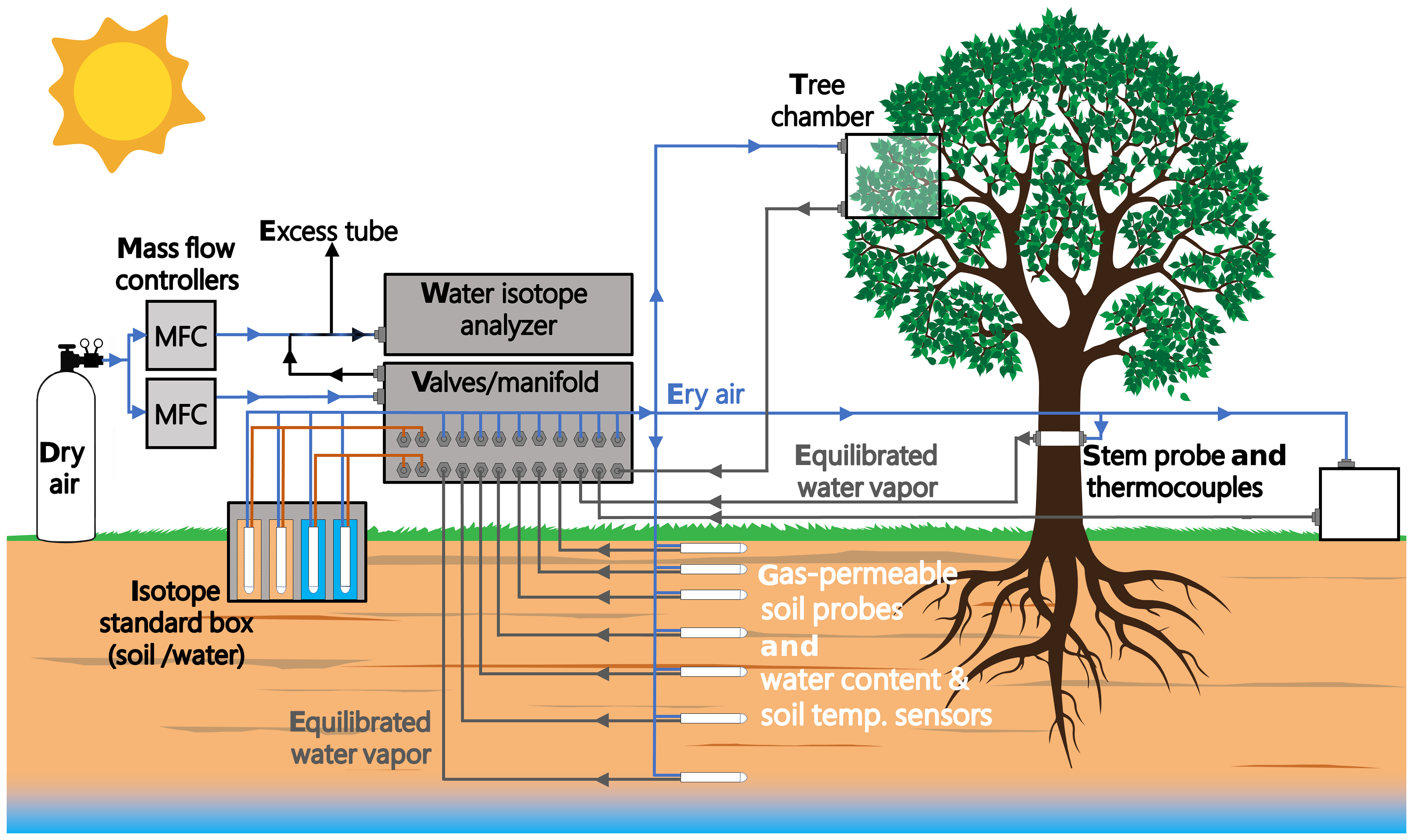



Post a Comment for "The Progress With Time Of A Reaction System Is Depicted"